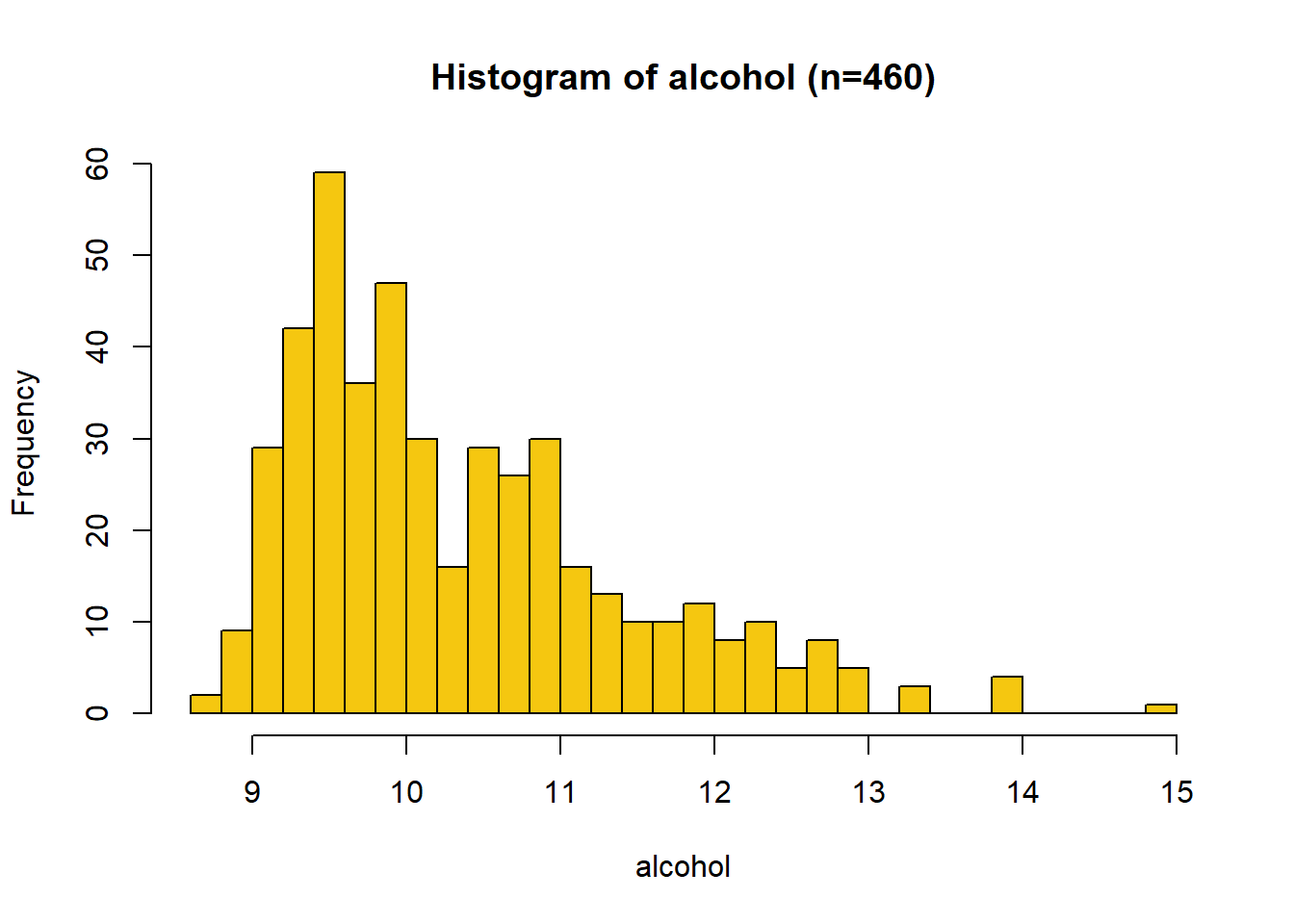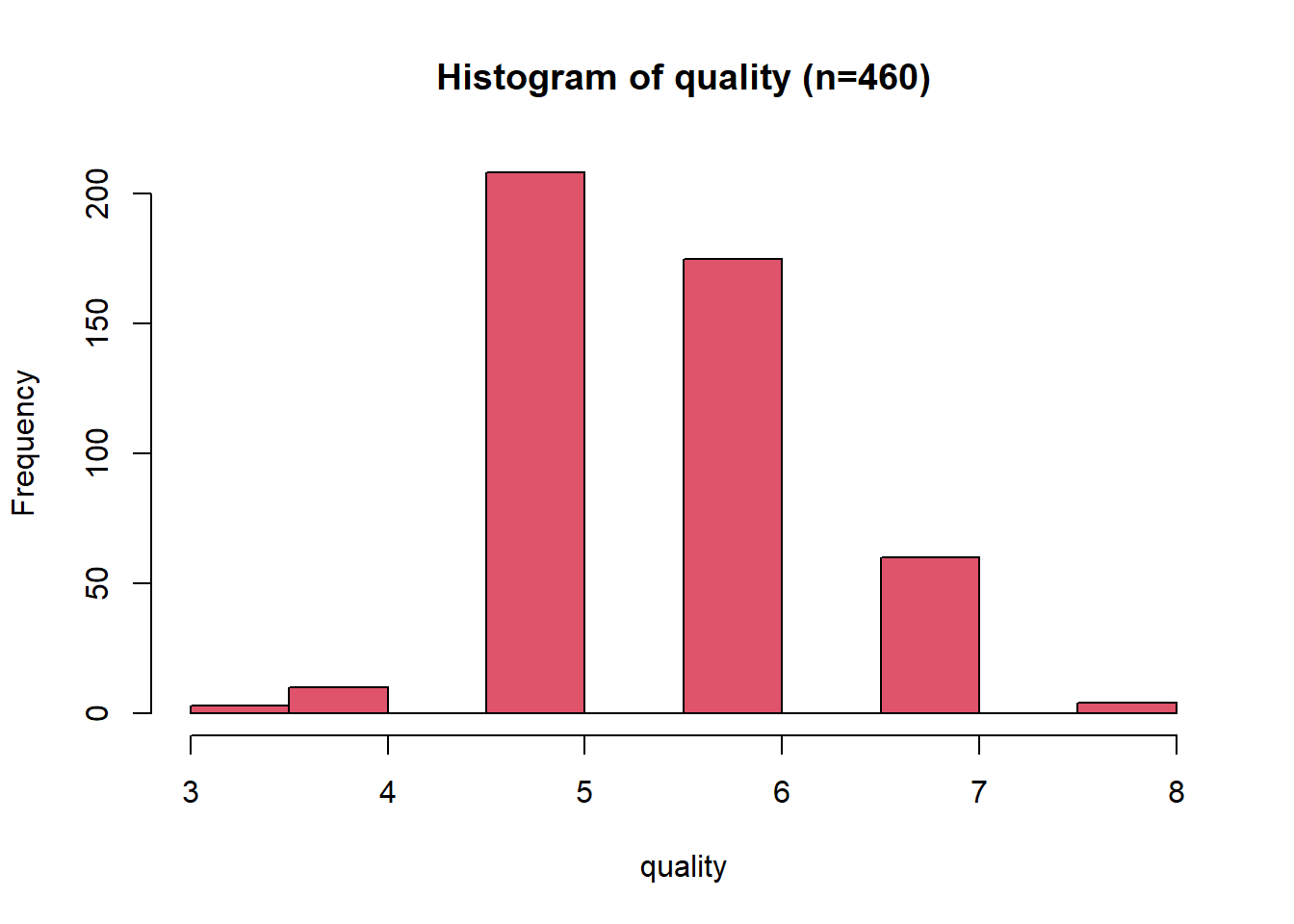#(i) and (ii)
data.raw = as.matrix(read.table("RedWine.txt"))
set.seed(225259172) # the seed is my student ID number
#(iii)
subset.num_samples = 460
data.subset = data.raw[sample(1:1599, subset.num_samples), c(1:6)]
data.variable.names = c("citric acid", "chlorides", "total sulfur dioxide", "pH", "alcohol")
y.name = 'quality'
#(iv)
# Create 5 scatterplots function (for each X variable against the variable of interest Y)"
colours = c("#9E9E9E","#61D04F","#2297E6","#CD0BBC","#F5C710","#DF536B")
for (i in c(1,2,3,4,5)){
name = data.variable.names[i]
plot(x = data.subset[,i], y = data.subset[,6], col = colours[i], pch=20, cex= 3, xlab= name, ylab=y.name,
main=sprintf("Scatter plot of %s versus %s (n=%s)", y.name, name, subset.num_samples)
)
}Red Wine
Using Aggregation Function for Data Analysis
Dataset: Red Portuguese “Vinho Verde” wine (Cortez et al., 2009)
The Data
- Dataset: Red Portuguese “Vinho Verde” wine (Cortez et al., 2009)
- Five input variables: citric acid, chlorides, total sulfur dioxide, pH, alcohol
- One output variable: quality
Data Exploration
This section uses scatter plots to explore the relationship between wine quality and the independent variables citric acid, chlorides, total sulfur dioxide, and alcohol. Histogram plots of the dependent and independent variables are used to explore the distribution of each variable.
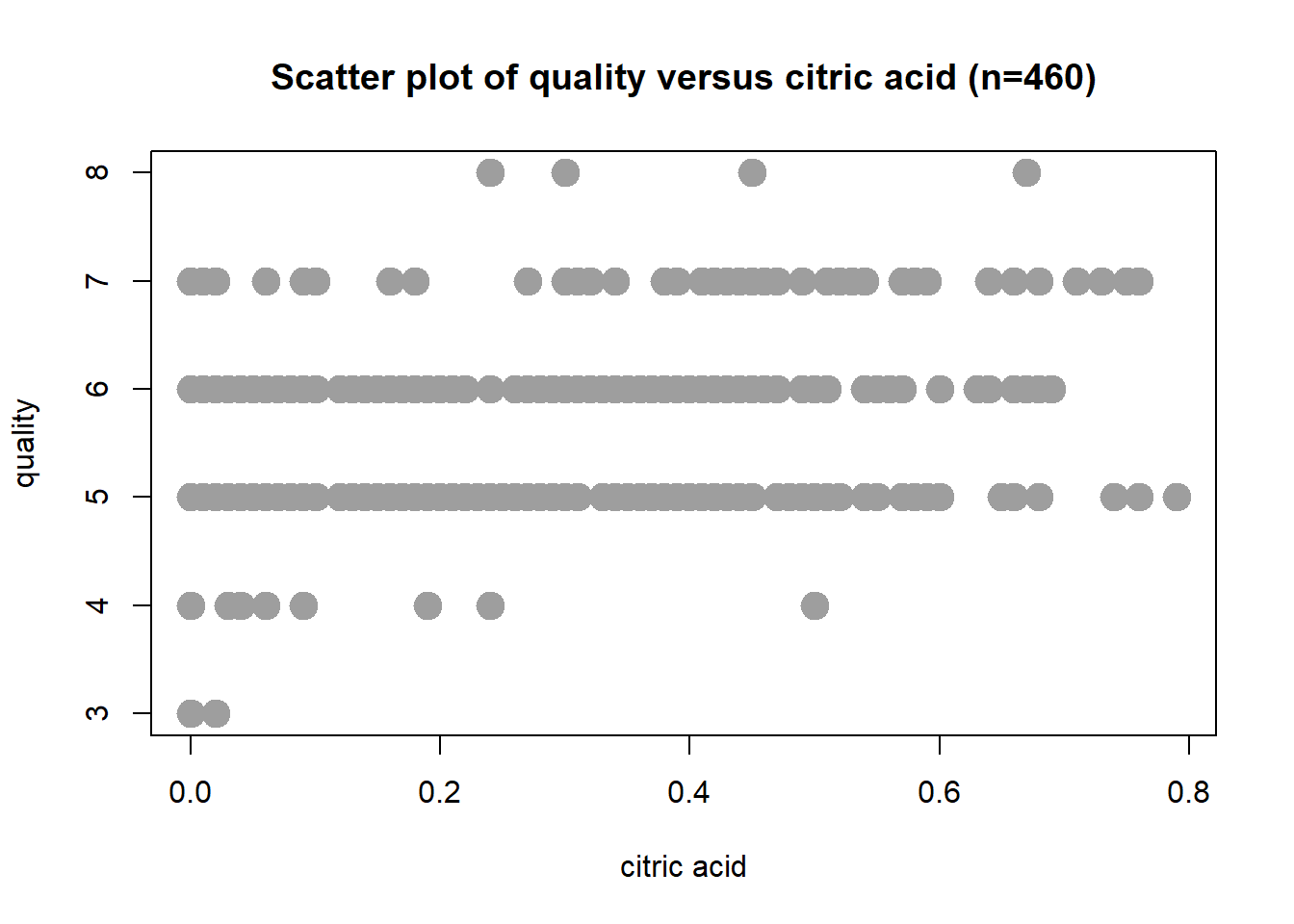
Scatter Plot: Citric Acid
Wine quality increases with increasing citric acid value. The correlation coefficient is r = 0.244.
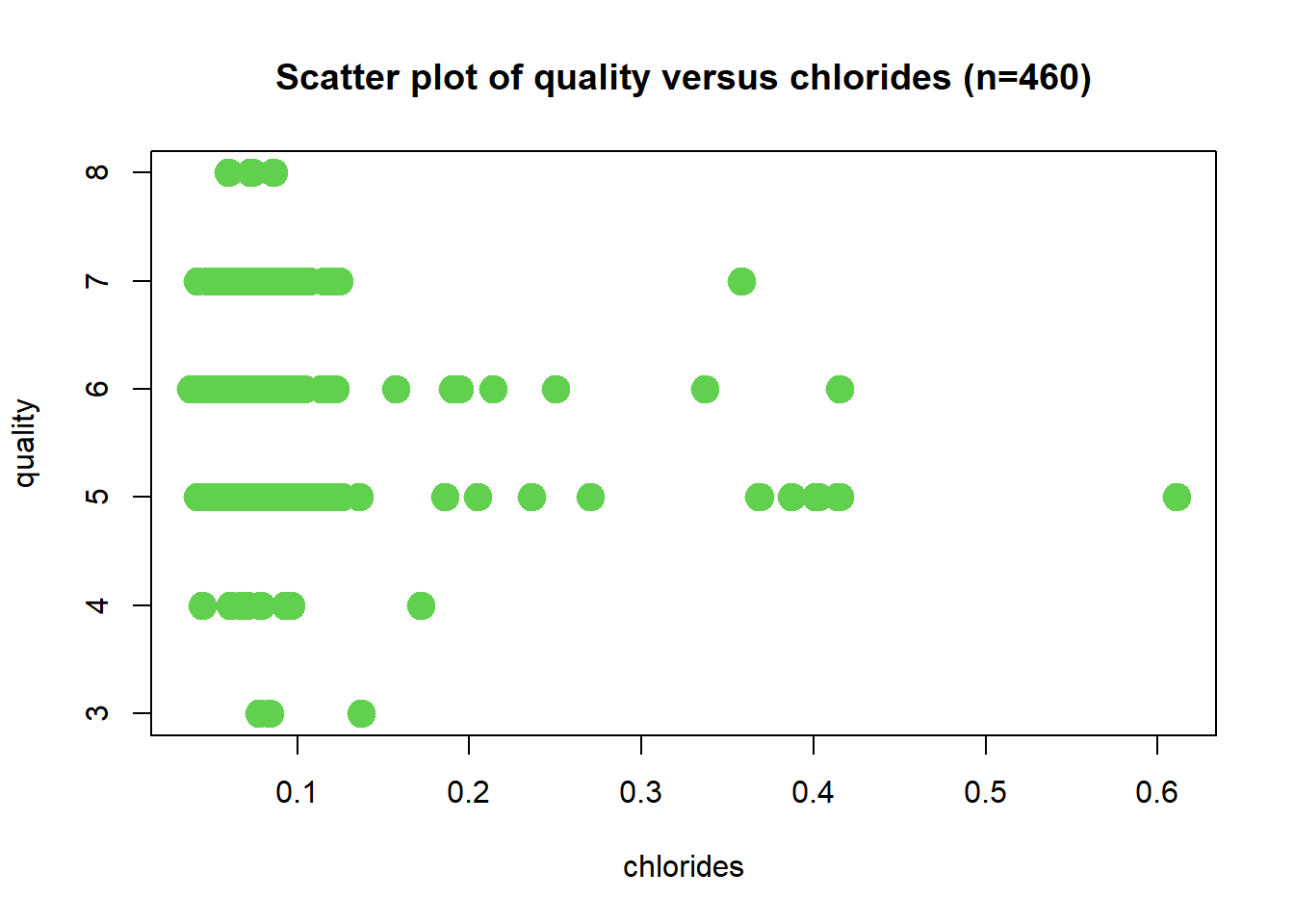
Scatter Plot: Chlorides
Wine quality decreases with increasing chlorides value. The correlation coefficient is r = -0.122. A potential outlier is observed at 0.61 chlorides value. A negation function will be required.
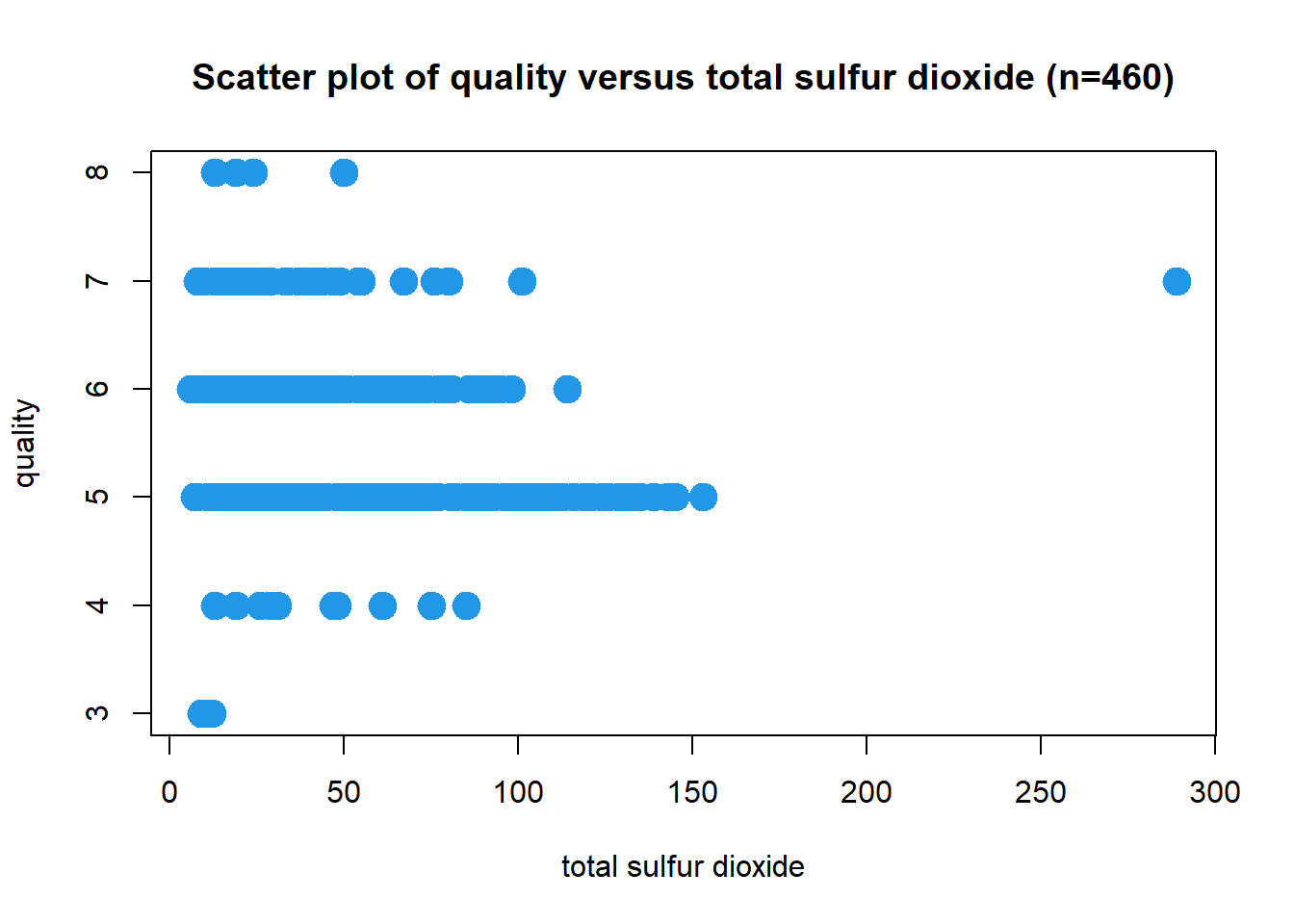
Scatter Plot: Total Sulfur Dioxide
Wine quality decreases with the increasing value of total sulphur dioxide. The correlation coefficient is r = -0.206. A possible outlier at a value of 289 for total sulfur dioxide. A negation function will be required.
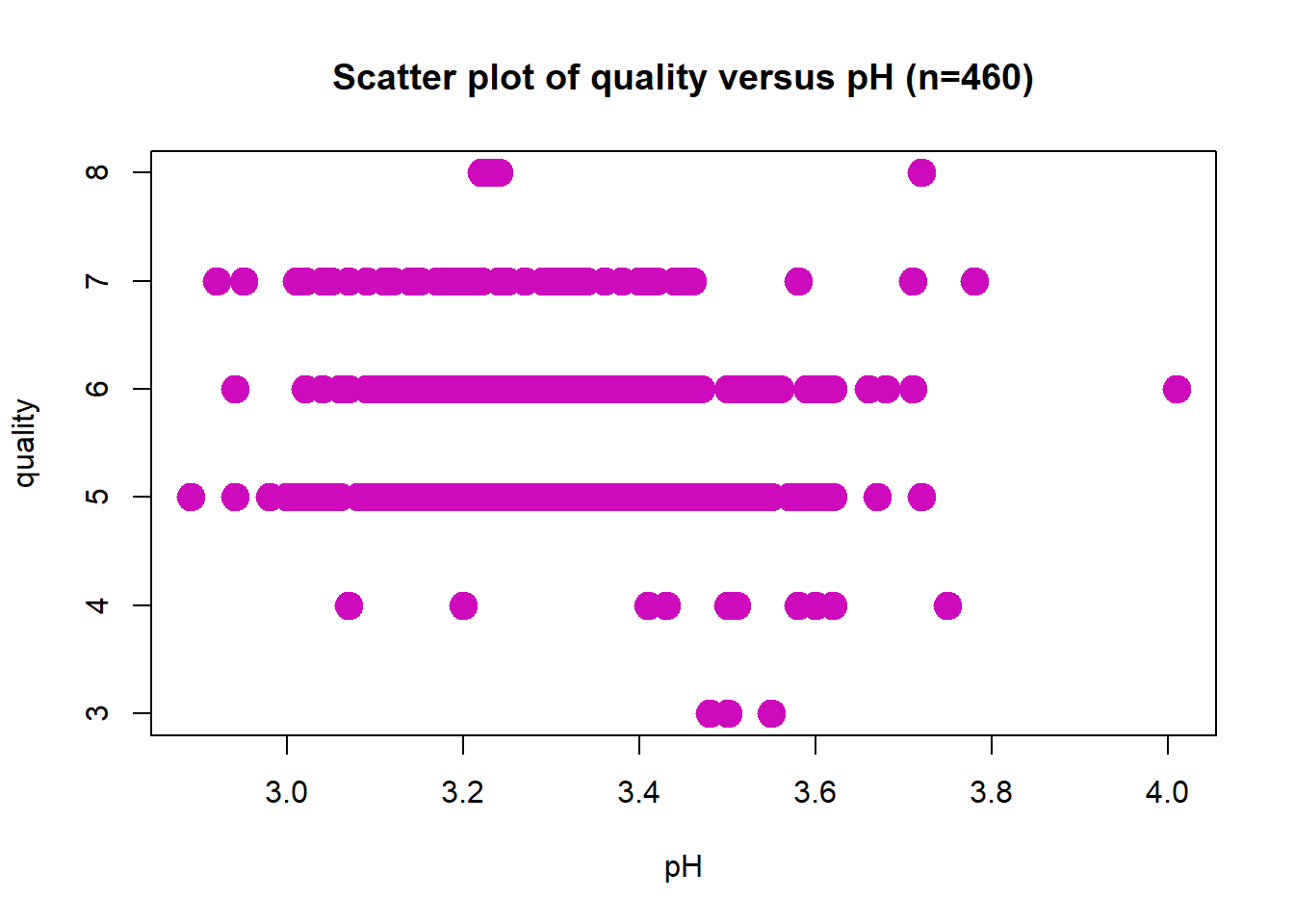
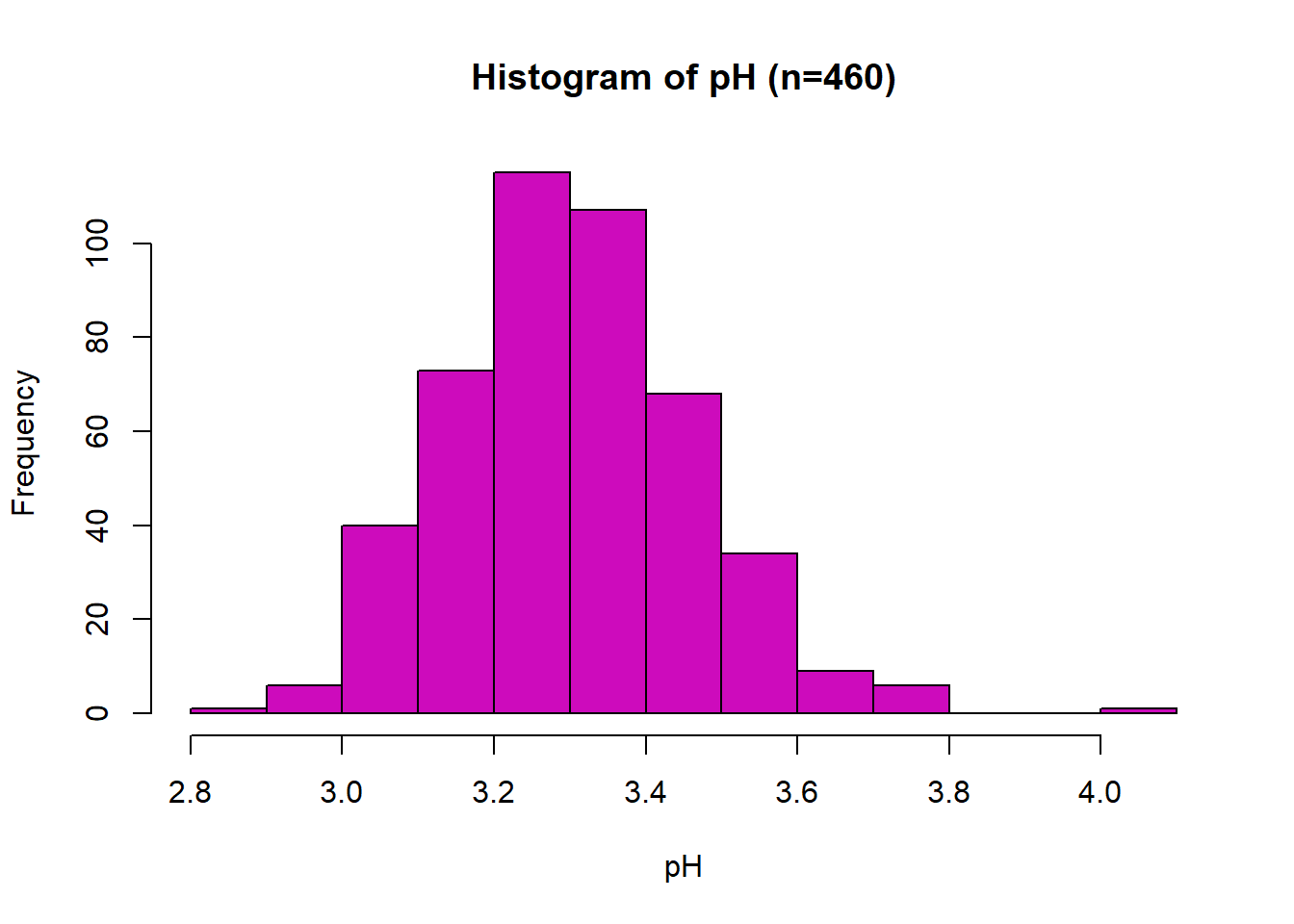
Scatter Plot: pH
Wine quality does not appear to have a linear relationship with pH. The correlation coefficient is r = -0.072. Potential outlier at pH value of 4.0.
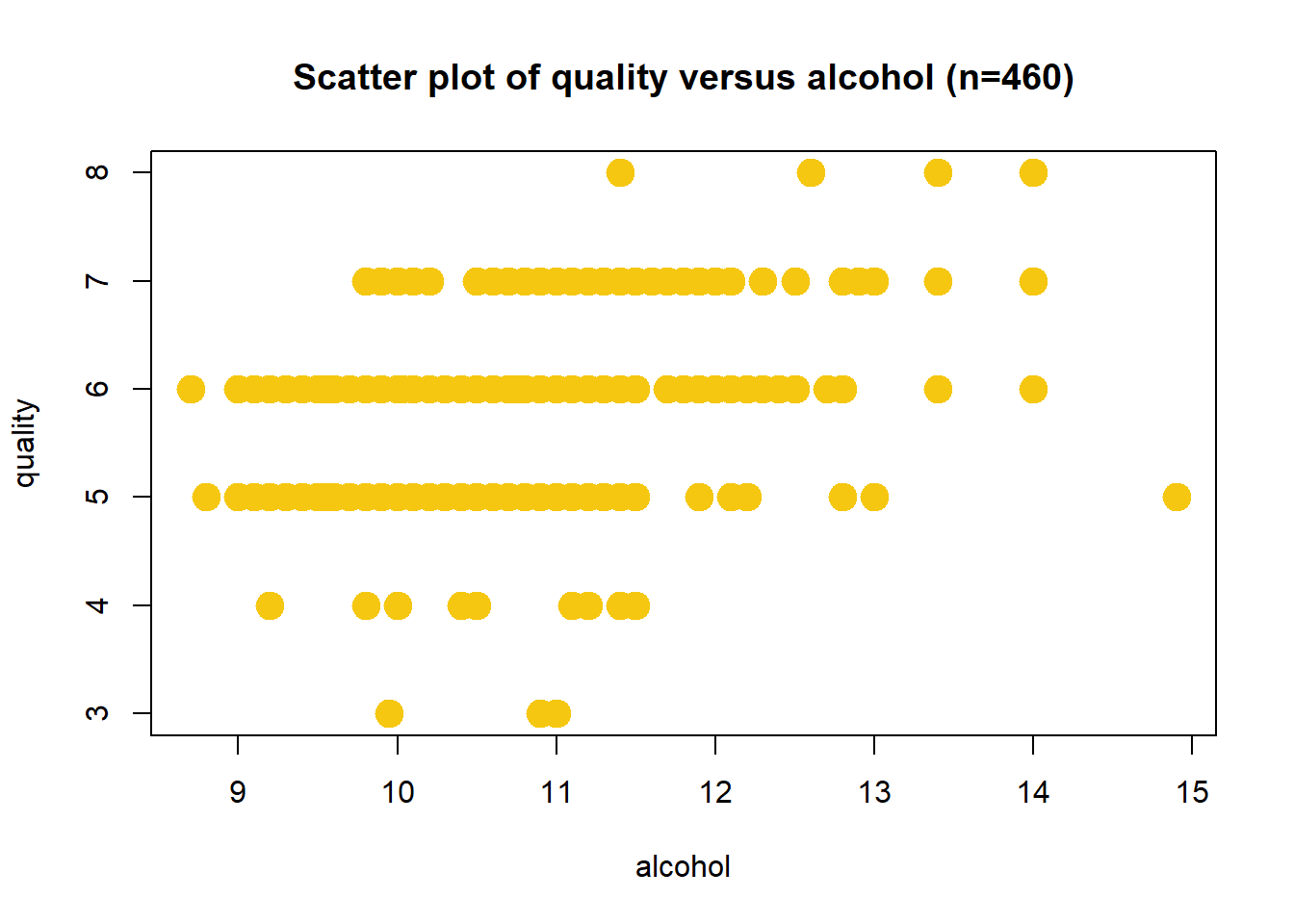
Scatter Plot: Alcohol
Wine quality increases with alcohol content. The correlation coefficient is r = 0.442.
# Number of bins for histograms
bins = c(16,30,48,11,24,8)
# Create 6 histograms for each X variable and Y
for (i in c(1,2,3,4,5,6)){
if (i == 6){
name = y.name
}
else{
name = data.variable.names[i]
}
hist(data.subset[,i], breaks=bins[i], xlab=name, main=sprintf("Histogram of %s (n=%s)", name, subset.num_samples),
col=colours[i]
)
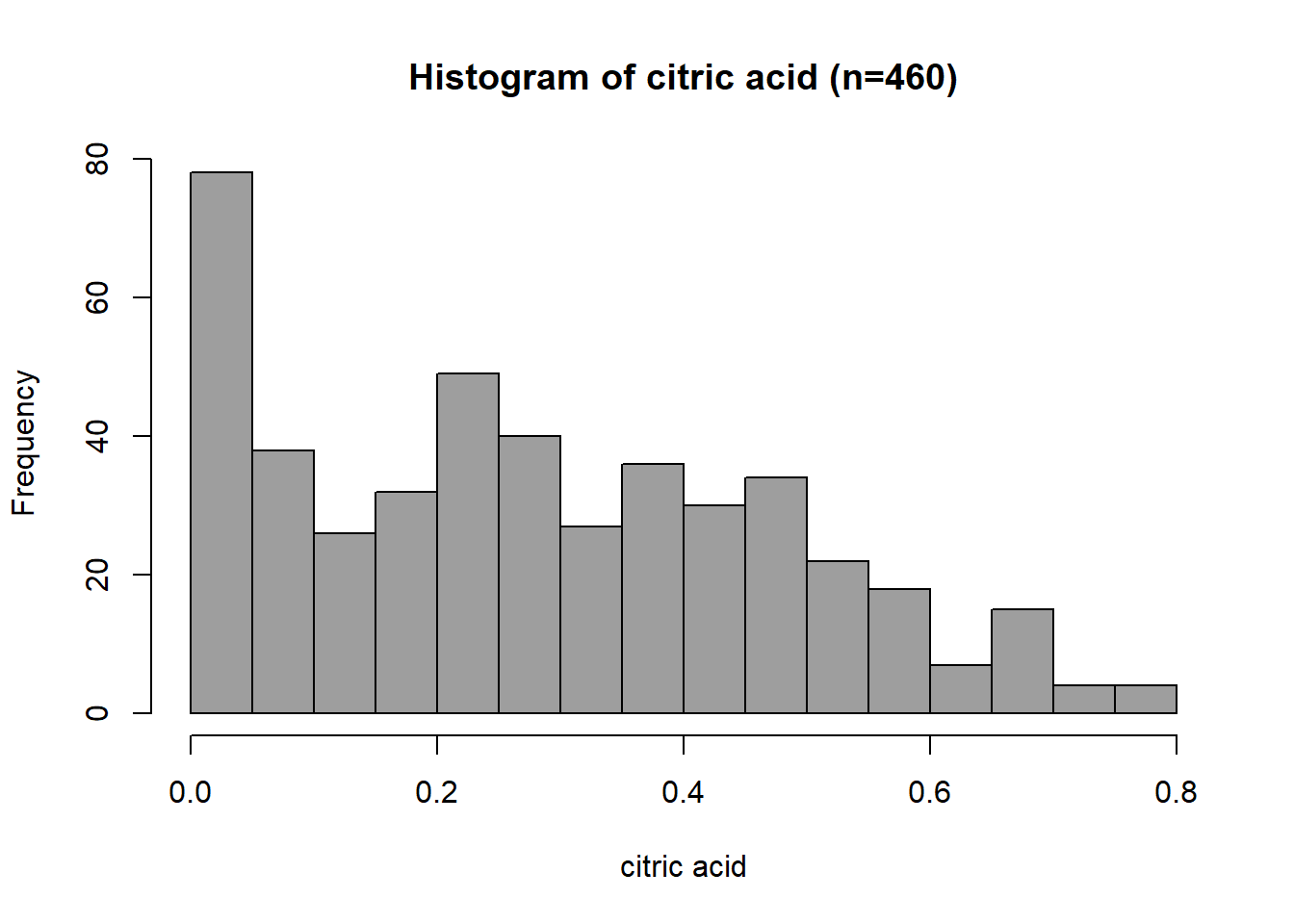
}Histogram: Citric Acid
The distribution of citric acid is uniform for the middle values. However, many observations show a peak near-zero value. At the higher citric acid values, fewer observations give the distribution a right (positive) skew. The distribution range is from 0.000 to 0.790, with a median value of 0.260 and a mean of 0.284.
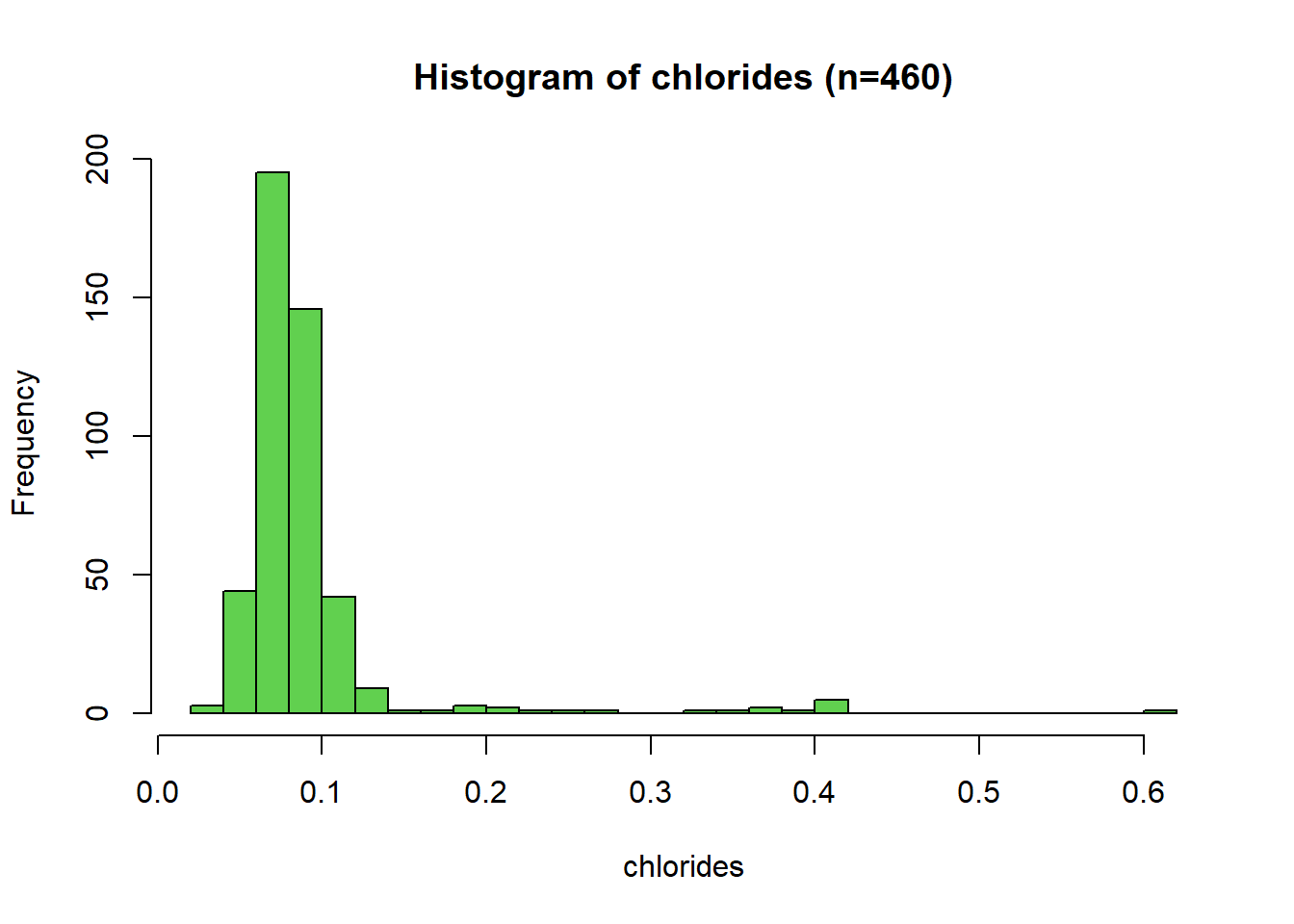
Histogram: Chlorides
The distribution of chlorides is unimodal with significant right (positive) skew. Most of the chlorides observations are between 0.04 and 0.15. Values above 0.15 have very few counts and are potential outliers. The distribution range is from 0.038 to 0.611, with a median value of 0.080 and a mean of 0.0912.
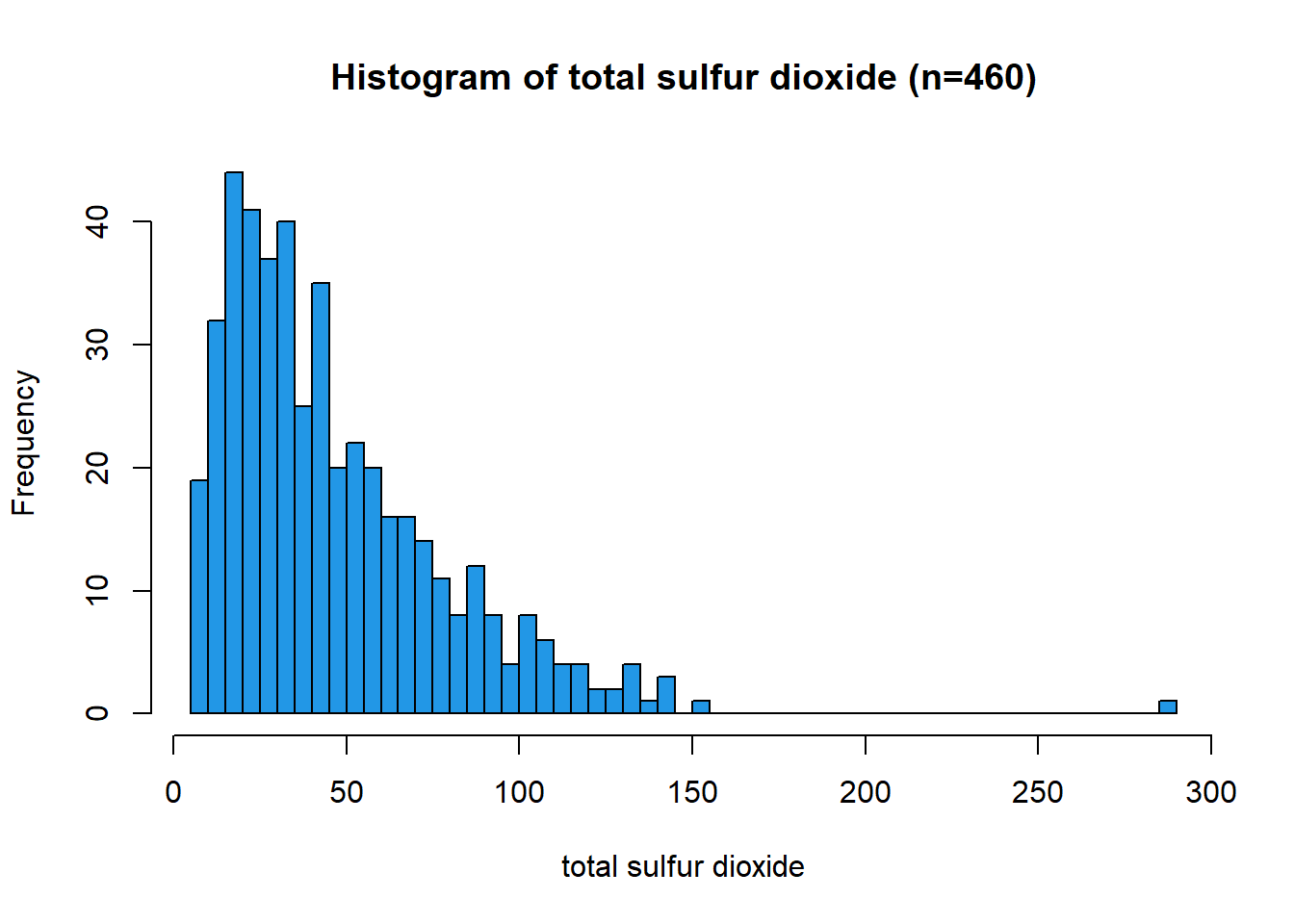
Histogram: Total Sulfur Dioxide
The distribution of total sulfur dioxide is unimodal with right (positive) skew and reassembles a log-normal shape. The value of 289 appears to be an outlier. The distribution range is from 6.0 to 289.0, with a median value of 38.5 and a mean of 47.12.
Histogram: pH
The distribution of pH is unimodal with minimal skew and a potential outlier at 4.01 and has a normal distribution shape. The distribution range is from 2.89 to 4.01, with a median value of 3.30 and a mean of 3.31.
Histogram: Alcohol
The distribution of alcohol is unimodal with right (positive) skew and reassembles a log-normal shape. It ranges from 8.7 to 14.9, with a median value of 10.1 and a mean of 10.4.
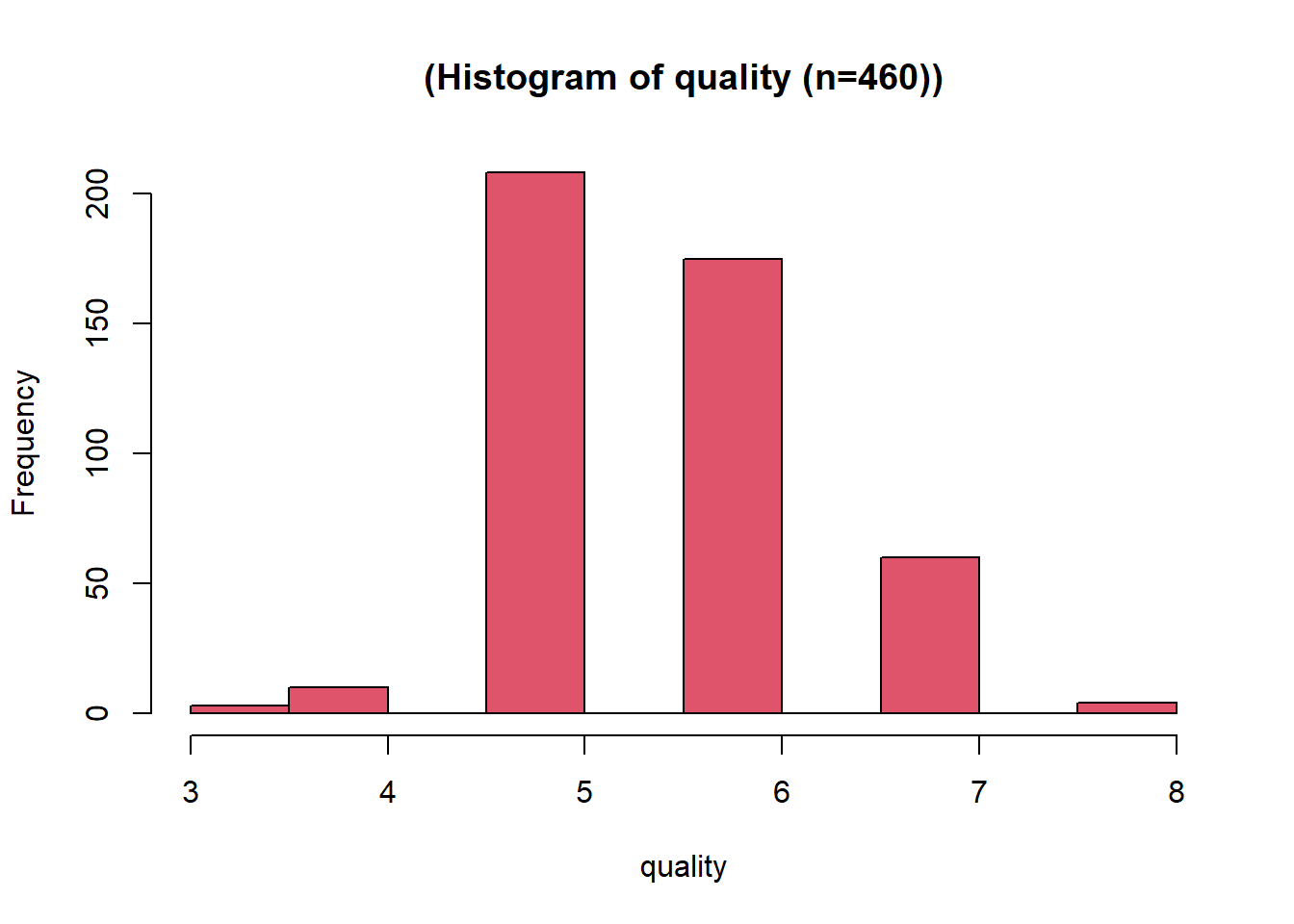
Histogram: Quality
The quality distribution is unimodal, with some right (positive) skew having a normal distribution shape. The data contains only integer values, which leads to gaps in the histogram unless a small number of bins are used. The distribution range is from 3 to 8, with a median value of 6 and a mean of 5.6.
# Generate a summary of the data
summary(data.subset) V1 V2 V3 V4
Min. :0.0000 Min. :0.03800 Min. : 6.00 Min. :2.890
1st Qu.:0.1000 1st Qu.:0.07100 1st Qu.: 23.00 1st Qu.:3.200
Median :0.2600 Median :0.08000 Median : 38.50 Median :3.300
Mean :0.2837 Mean :0.09117 Mean : 47.12 Mean :3.308
3rd Qu.:0.4400 3rd Qu.:0.09400 3rd Qu.: 64.25 3rd Qu.:3.410
Max. :0.7900 Max. :0.61100 Max. :289.00 Max. :4.010
V5 V6
Min. : 8.70 Min. :3.000
1st Qu.: 9.50 1st Qu.:5.000
Median :10.10 Median :6.000
Mean :10.41 Mean :5.633
3rd Qu.:11.00 3rd Qu.:6.000
Max. :14.90 Max. :8.000 # Calculate Pearson correlation coefficients
cor(data.subset, data.subset[,6]) [,1]
V1 0.24381427
V2 -0.12184464
V3 -0.20584651
V4 -0.07241671
V5 0.44197926
V6 1.00000000Transform the Data
The independent variables citric acid, chlorides, total sulfur dioxide, and alcohol were chosen for the aggregation function fitting. These variables had the strongest association with wine quality. Treatment of outliers was considered, and it was decided not to apply any treatment.
library(e1071) # Required for Skewness function
library(rcompanion) # Provides plotting of normal PDF on histogram
library(latex2exp) # Provides the ability to use latex in plot titles/labels
# min-max normalisation
# Allows optional arguements to set the min and max for the scaling
minmax = function(x, x_min=NULL, x_max=NULL){
if (is.null(x_min)){
x_min = min(x)
}
if (is.null(x_max)){
x_max = max(x)
}
(x - x_min)/(x_max-x_min)
}
# z-score standardisation and scaling to unit interval
unit.z = function(x){
0.15*((x-mean(x))/sd(x)) + 0.5
}
# Negation function for normalised data
negation = function(x){
(max(x) + min(x)) - x
}
# Vector with indices of chosen variables
I = c(1,2,3,5,6)
# Matrix containing the variables to transform
variables_for_transform = data.subset[,I]
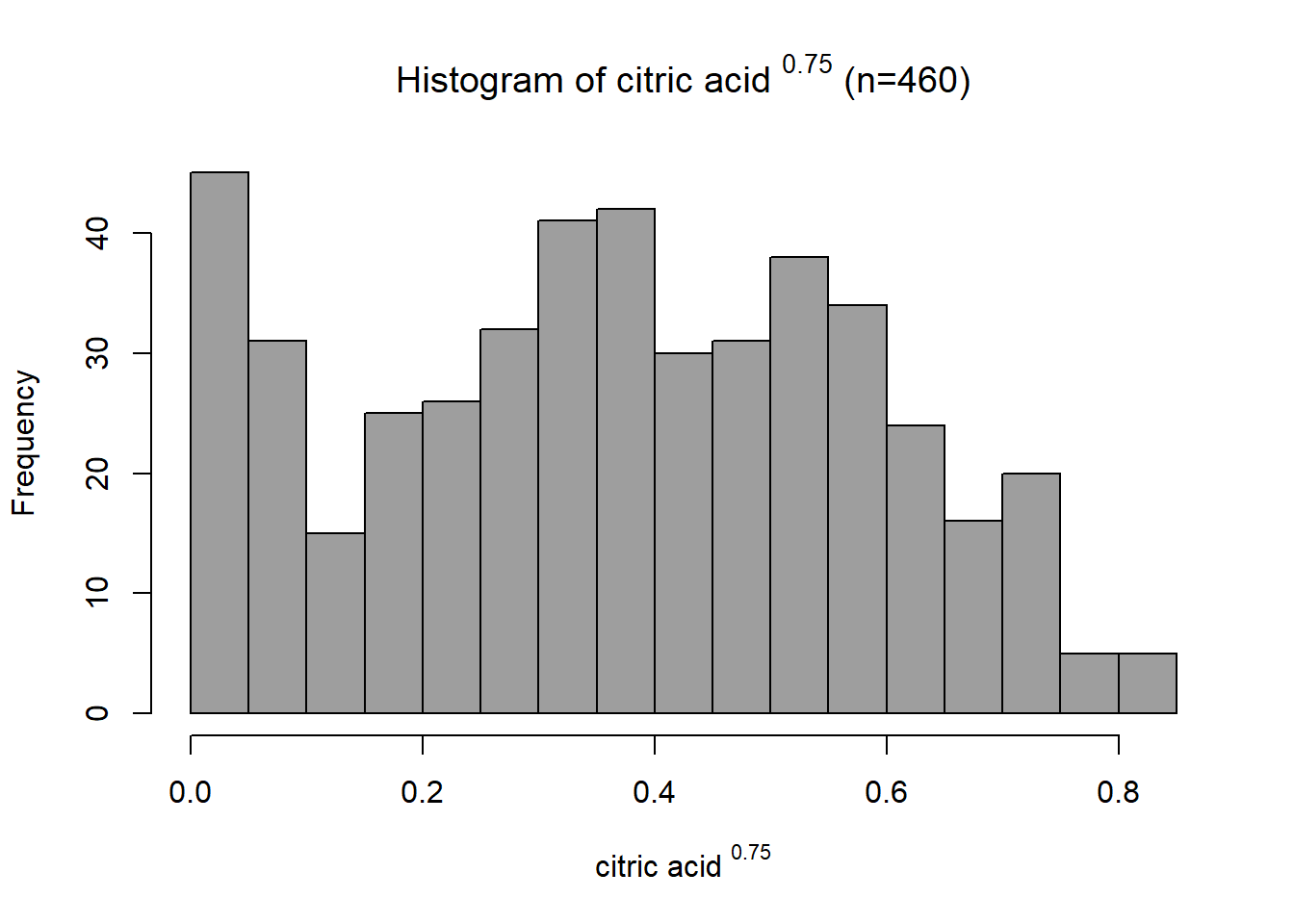
data.transformed = variables_for_transformCitric acid
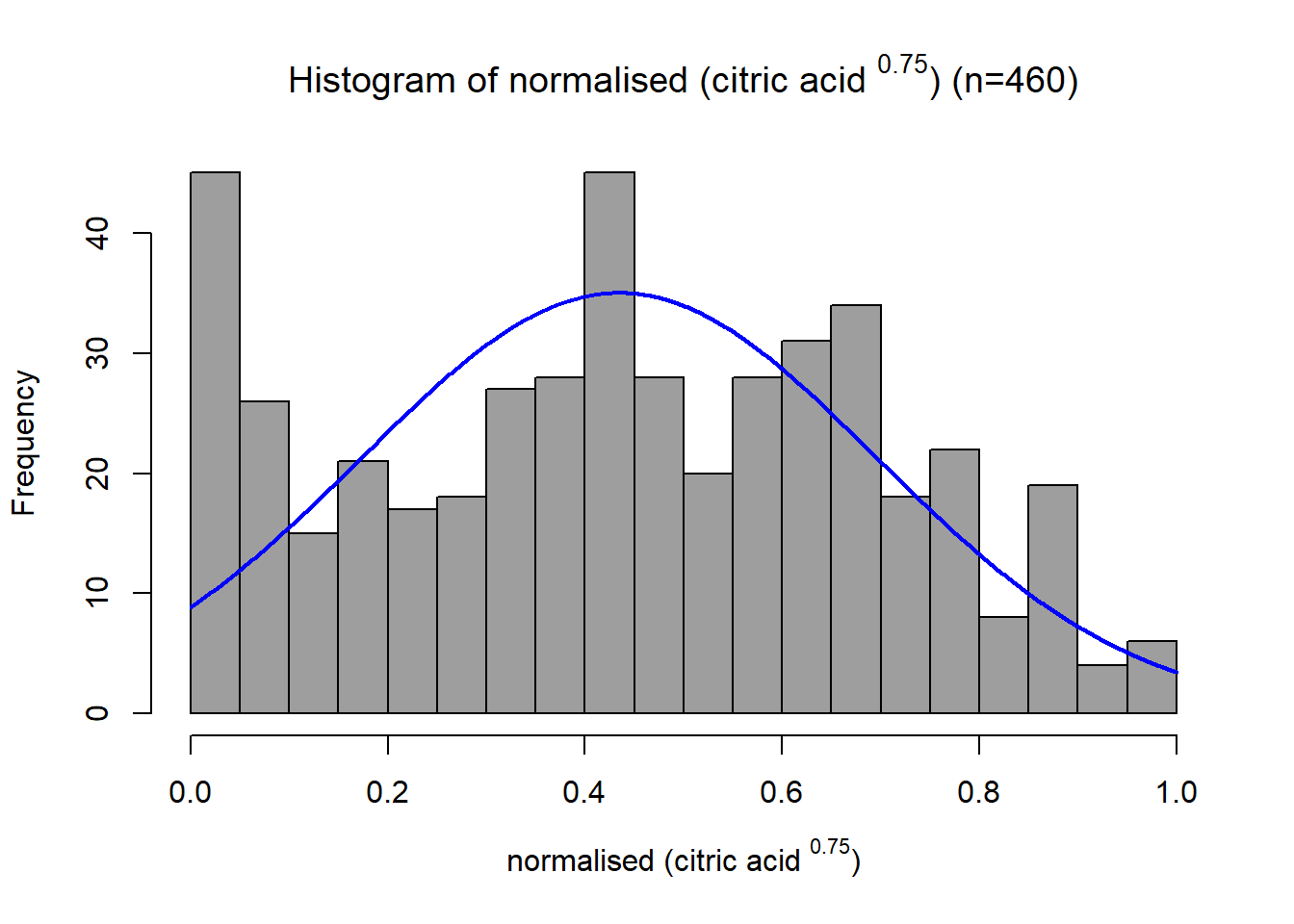
This distribution has some right skew with a skewness value of 0.326. Since the data is right-skewed, a power or log transformation is suitable. Through trial and error, the final transformation applied was \[x_{new} = x^{0.75}\]
This transformed the distribution closer to a normal distribution with a skewness of -0.04. The variable was then scaled between 0 and 1 using a min-max function. This variable already had a positive association with quality, so no negation was required.
# Transform
p=0.75 # final value after trying p=0.5 first
data.transformed[,1] = variables_for_transform[,1]^p
skewness(data.transformed[,1])[1] -0.0399249name1 = TeX(sprintf(r"(%s $^{%s}$)", data.variable.names[I[1]], p))
title1 = TeX(sprintf(r"(Histogram of %s $^{%s}$ (n=%s))", data.variable.names[I[1]], p, subset.num_samples))
hist(data.transformed[,1],xlab=name1, breaks=bins[I[1]], main=title1, col=colours[I[1]])min_1 = min(data.transformed[,1])
max_1 = max(data.transformed[,1])
# Normalise between 0 - 1
data.transformed[,1] = minmax(data.transformed[,1])
name1s = TeX(sprintf(r"(normalised (%s $^{%s}$))", data.variable.names[I[1]], p))
title1s = TeX(sprintf(r"(Histogram of normalised (%s $^{%s}$) (n=%s))", data.variable.names[I[1]], p, subset.num_samples))
plotNormalHistogram(data.transformed[,1], xlab=name1s, breaks=bins[I[1]], main=title1s, col=colours[I[1]],xlim=c(0,1))Chlorides
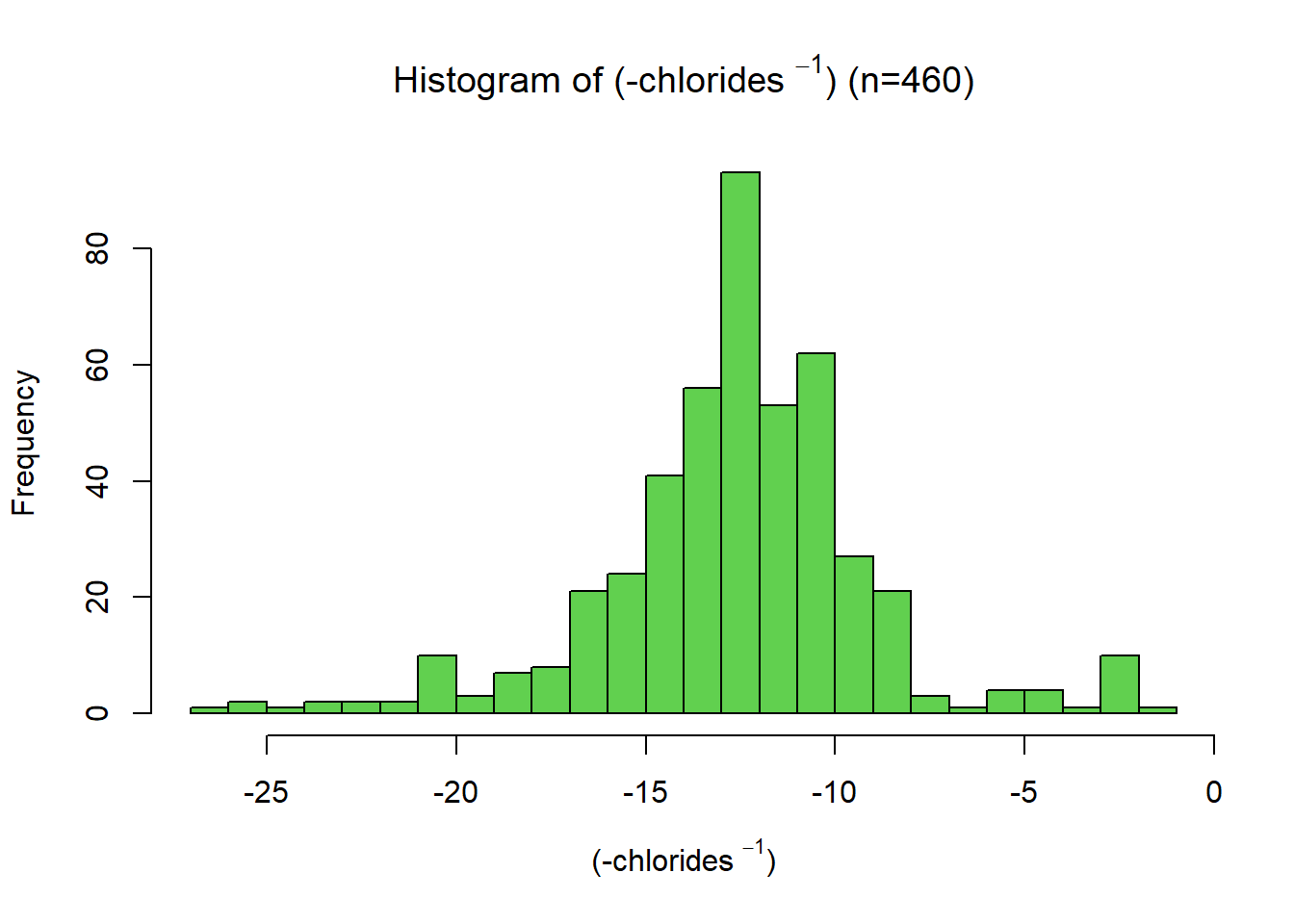
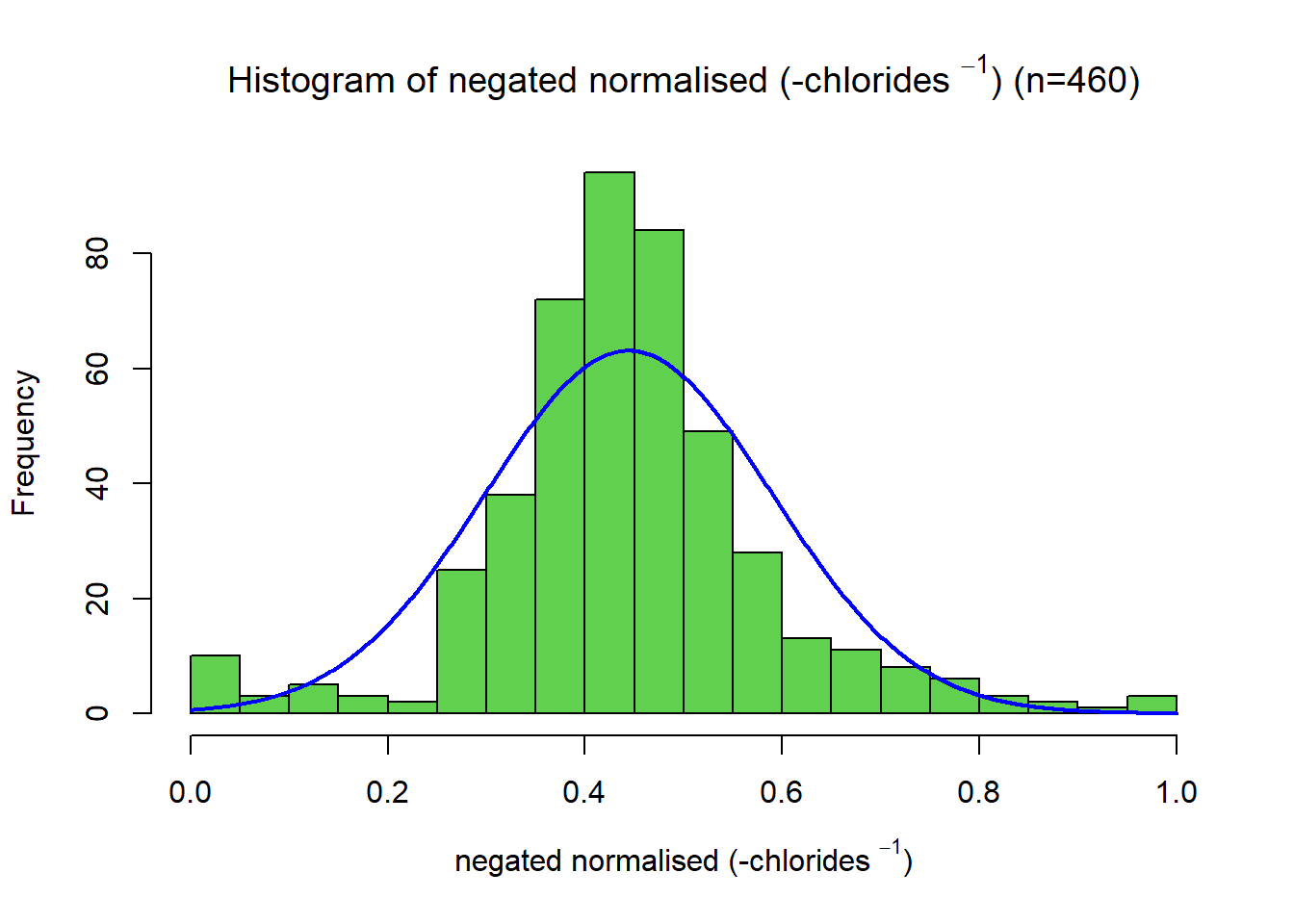
This distribution has a significant right skew, with a skewness value of 5.0. Since the data is right-skewed, a suitable transformation is a power or log transformation. A more substantial transformation than the log was required. Through trial and error, the final transformation applied was \[ x_{new} = -\frac{1}{x}\] Giving a skewness of -0.259.The variable was then scaled between 0 and 1 using a min-max function. This variable had a negative association with quality, so a negation function was applied.
# Transform
p=-1 # final value after trying log transform first
data.transformed[,2] = -variables_for_transform[,2]^p
skewness(data.transformed[,2])[1] -0.2587889name2 = TeX(sprintf(r"((-%s $^{%s}$))", data.variable.names[I[2]], p))
title2 = TeX(sprintf(r"(Histogram of (-%s $^{%s}$) (n=%s))", data.variable.names[I[2]], p, subset.num_samples))
hist(data.transformed[,2],xlab=name2, breaks=bins[I[2]], main=title2, col=colours[I[2]])min_2 = min(data.transformed[,2])
max_2 = max(data.transformed[,2])
# Normalise between 0 - 1
data.transformed[,2] = minmax(data.transformed[,2])
name2s = TeX(sprintf(r"(negated normalised (-%s $^{%s}$))", data.variable.names[I[2]], p))
title2s = TeX(sprintf(r"(Histogram of negated normalised (-%s $^{%s}$) (n=%s))", data.variable.names[I[2]], p, subset.num_samples))
#plotNormalHistogram(data.transformed[,2], xlab=name2s, breaks=bins[I[2]], main=title2s, col=colours[I[2]],xlim=c(0,1))
# Negate
data.transformed[,2] = negation(data.transformed[,2])
plotNormalHistogram(data.transformed[,2], xlab=name2s, breaks=bins[I[2]], main=title2s, col=colours[I[2]],xlim=c(0,1))Total Sulfur Dioxide
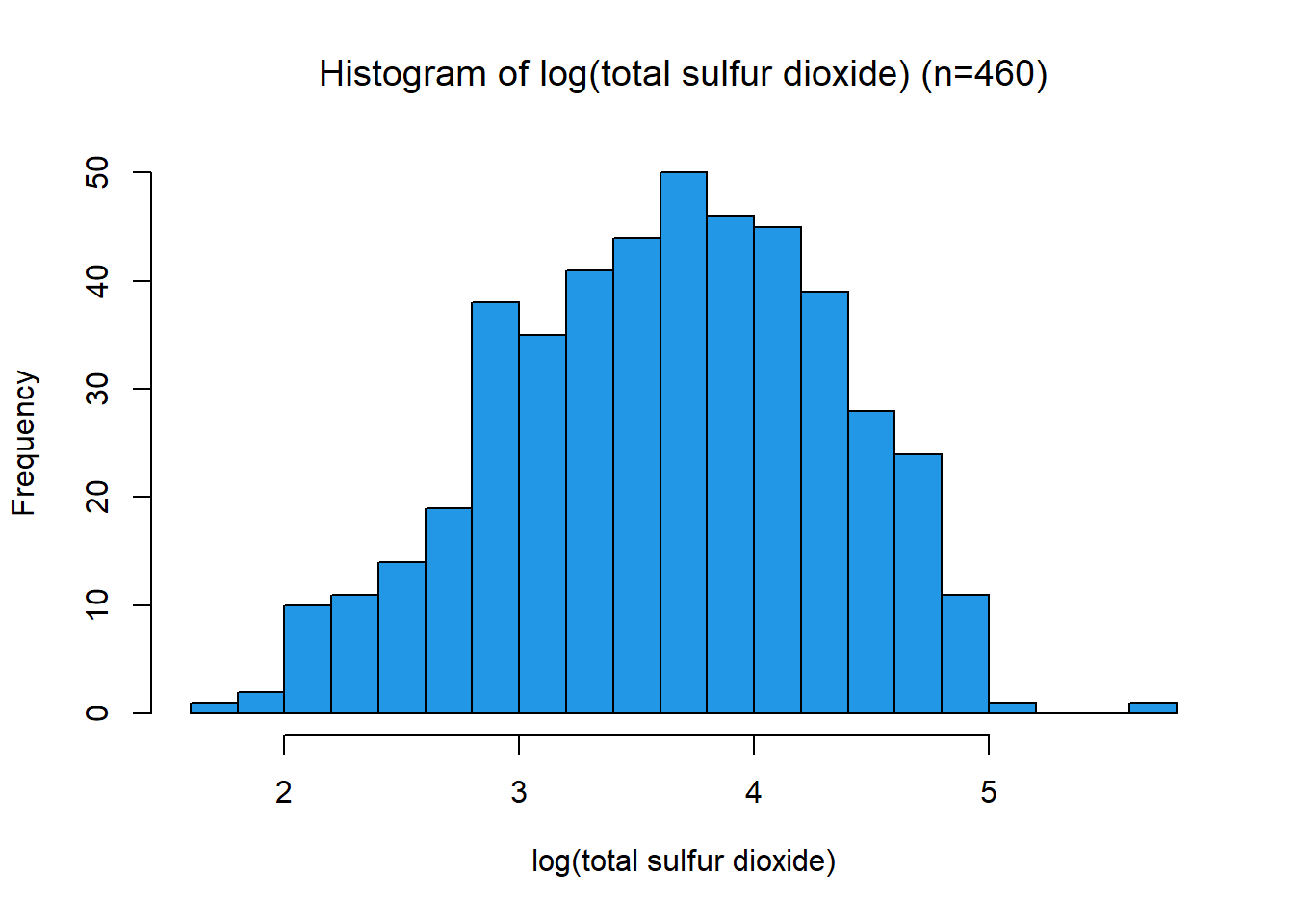
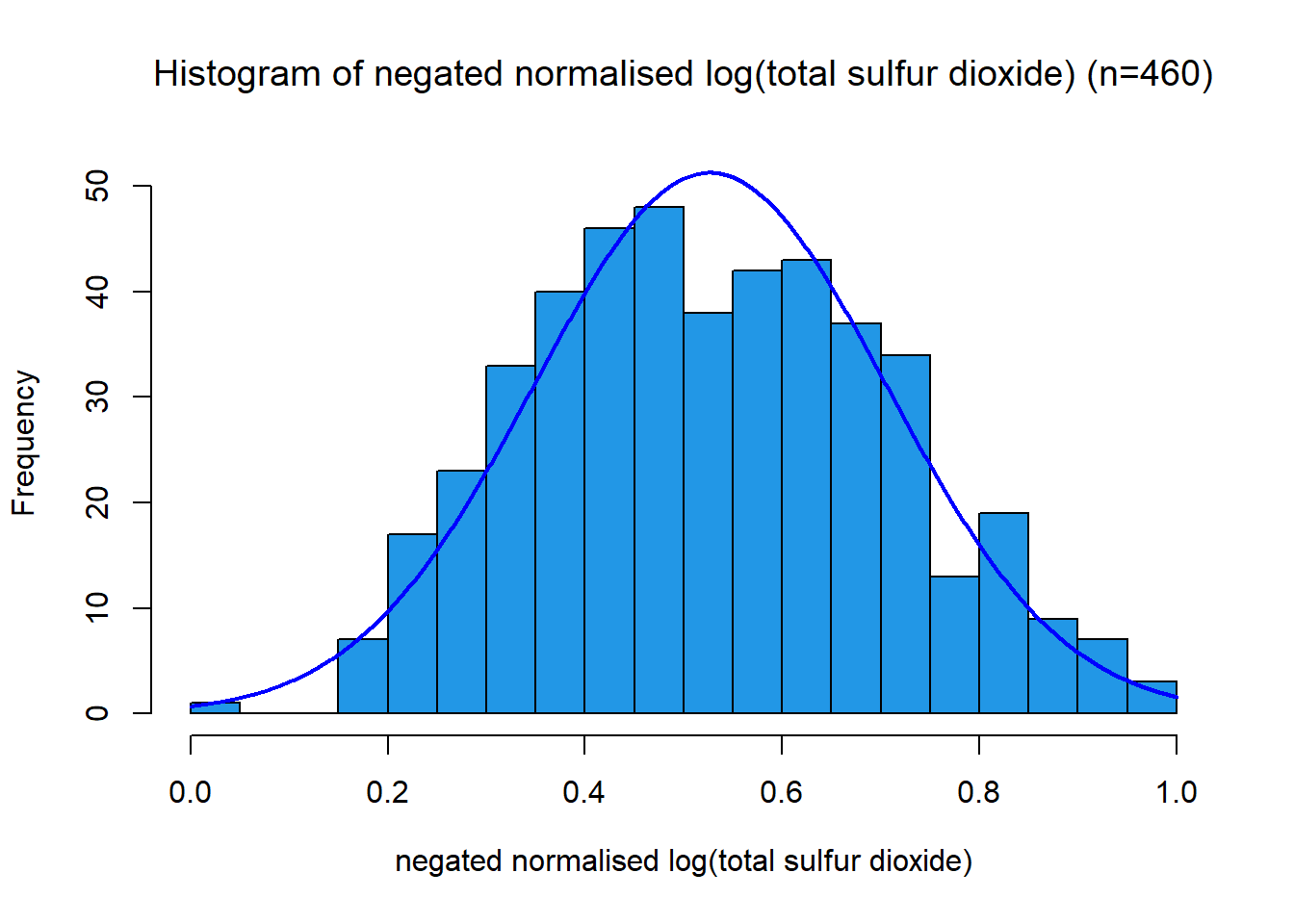
This distribution has a strong right skew, with a skewness value 1.72. Since the data is right-skewed, a suitable transformation is a power or log transformation. The distribution looks log-normal, the transformation applied was \[x_{new} = \log(x)\] This pushed the distribution closer to normal and reduced the skewness to -0.17. The variable was then scaled between 0 and 1 using a min-max function. This variable had a negative association with quality, so a negation function was applied.
[1] -0.1698297name3 = TeX(sprintf(r"(log(%s))", data.variable.names[I[3]]))
title3 = TeX(sprintf(r"(Histogram of log(%s) (n=%s))", data.variable.names[I[3]], subset.num_samples))
hist(data.transformed[,3],xlab=name3, breaks=24, main=title3, col=colours[I[3]])min_3 = min(data.transformed[,3])
max_3 = max(data.transformed[,3])
# Normalize between 0 - 1
data.transformed[,3] = minmax(data.transformed[,3])
# Negate
data.transformed[,3] = negation(data.transformed[,3])
name3s = TeX(sprintf(r"(negated normalised log(%s))", data.variable.names[I[3]]))
title3s = TeX(sprintf(r"(Histogram of negated normalised log(%s) (n=%s))", data.variable.names[I[3]], subset.num_samples))
plotNormalHistogram(data.transformed[,3], xlab=name3s, breaks=24, main=title3s, col=colours[I[3]],xlim=c(0,1))Alcohol
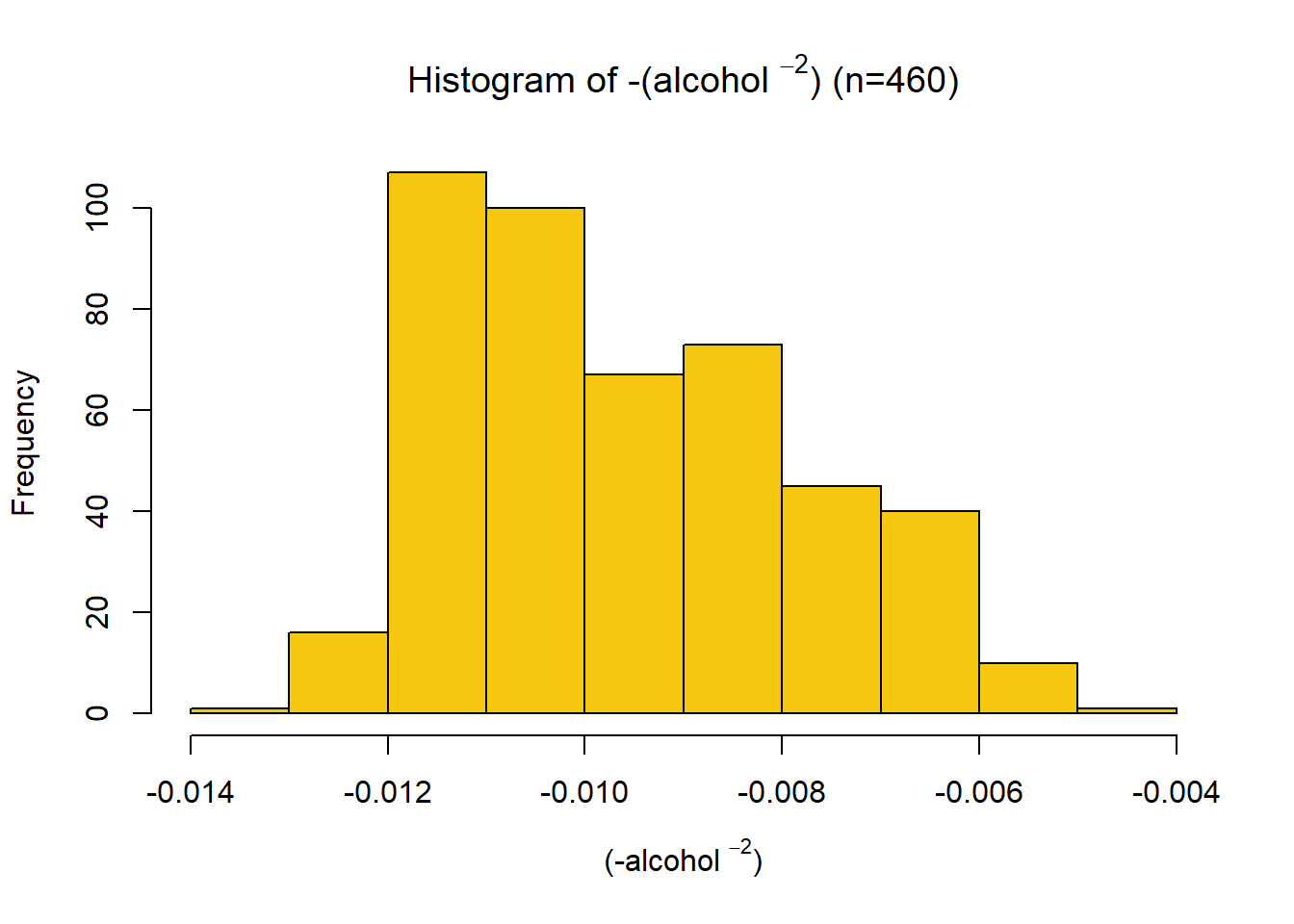
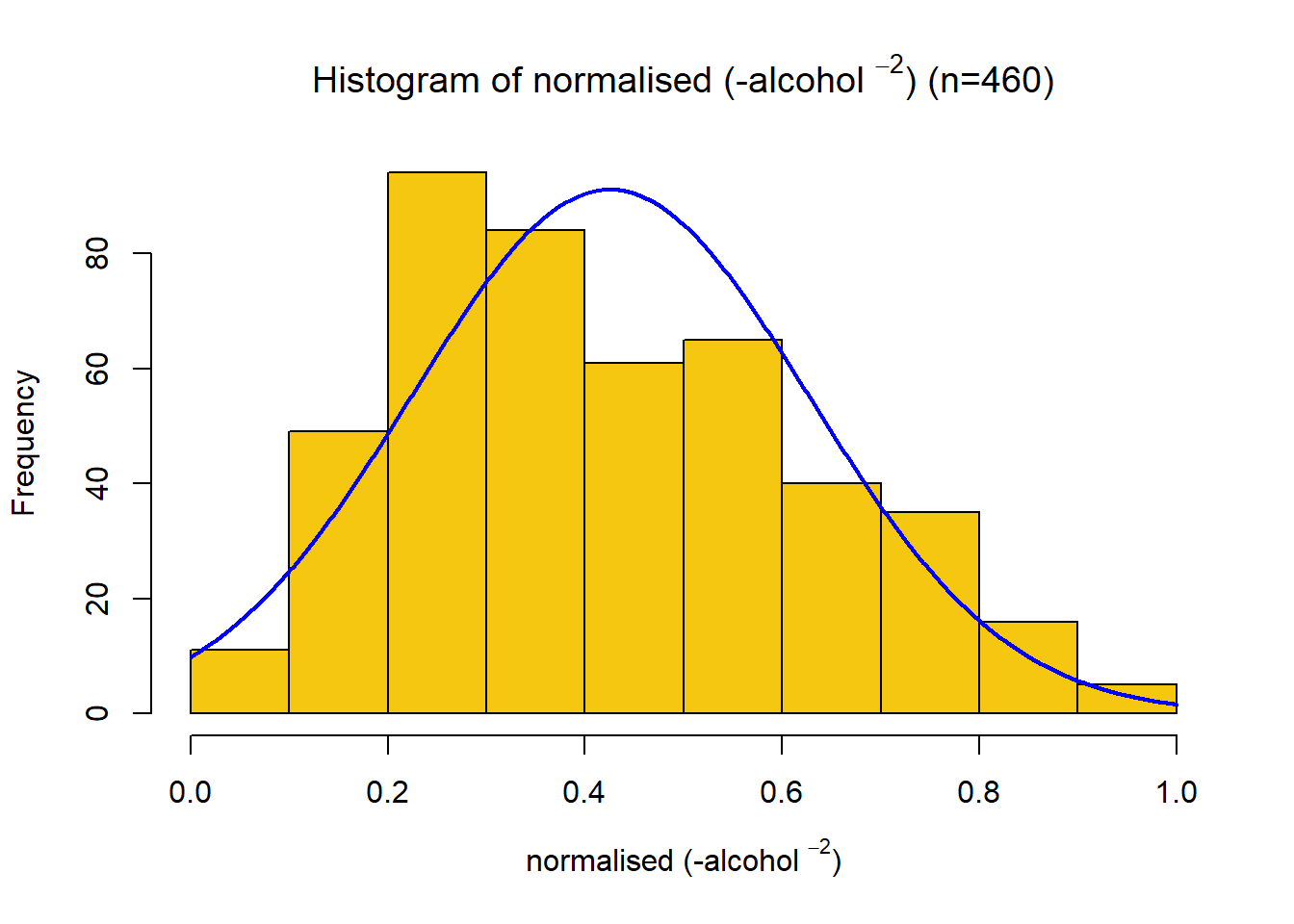
This distribution has a strong right skew, with a skewness value of 1.06. Since the data is right-skewed, a suitable transformation is a power or log transformation. The distribution looks log-normal, so a log transform was first tried. Through trial and error, the final transformation applied was \[x_{new} = -\frac{1}{x^2} \] Giving a skewness of -0.44. The variable was then scaled between 0 and 1 using a min-max function. This variable had a positive association with quality, so no negation was required.
# Transform
p=-2 # final value
data.transformed[,4] = -(variables_for_transform[,4]^p)
skewness(data.transformed[,4])[1] 0.4390636name4 = TeX(sprintf(r"((-%s $^{%s}$))", data.variable.names[I[4]],p))
title4 = TeX(sprintf(r"(Histogram of -(%s $^{%s}$) (n=%s))", data.variable.names[I[4]], p, subset.num_samples))
hist(data.transformed[,4],xlab=name4, breaks=12, main=title4, col=colours[I[4]])min_4 = min(data.transformed[,4])
max_4 = max(data.transformed[,4])
# Normalize between 0 - 1
data.transformed[,4] = minmax(data.transformed[,4])
name4s = TeX(sprintf(r"(normalised (-%s $^{%s}$))", data.variable.names[I[4]],p))
title4s = TeX(sprintf(r"(Histogram of normalised (-%s $^{%s}$) (n=%s))", data.variable.names[I[4]], p, subset.num_samples))
plotNormalHistogram(data.transformed[,4], xlab=name4s, breaks=12, main=title4s, col=colours[I[4]],xlim=c(0,1))Quality
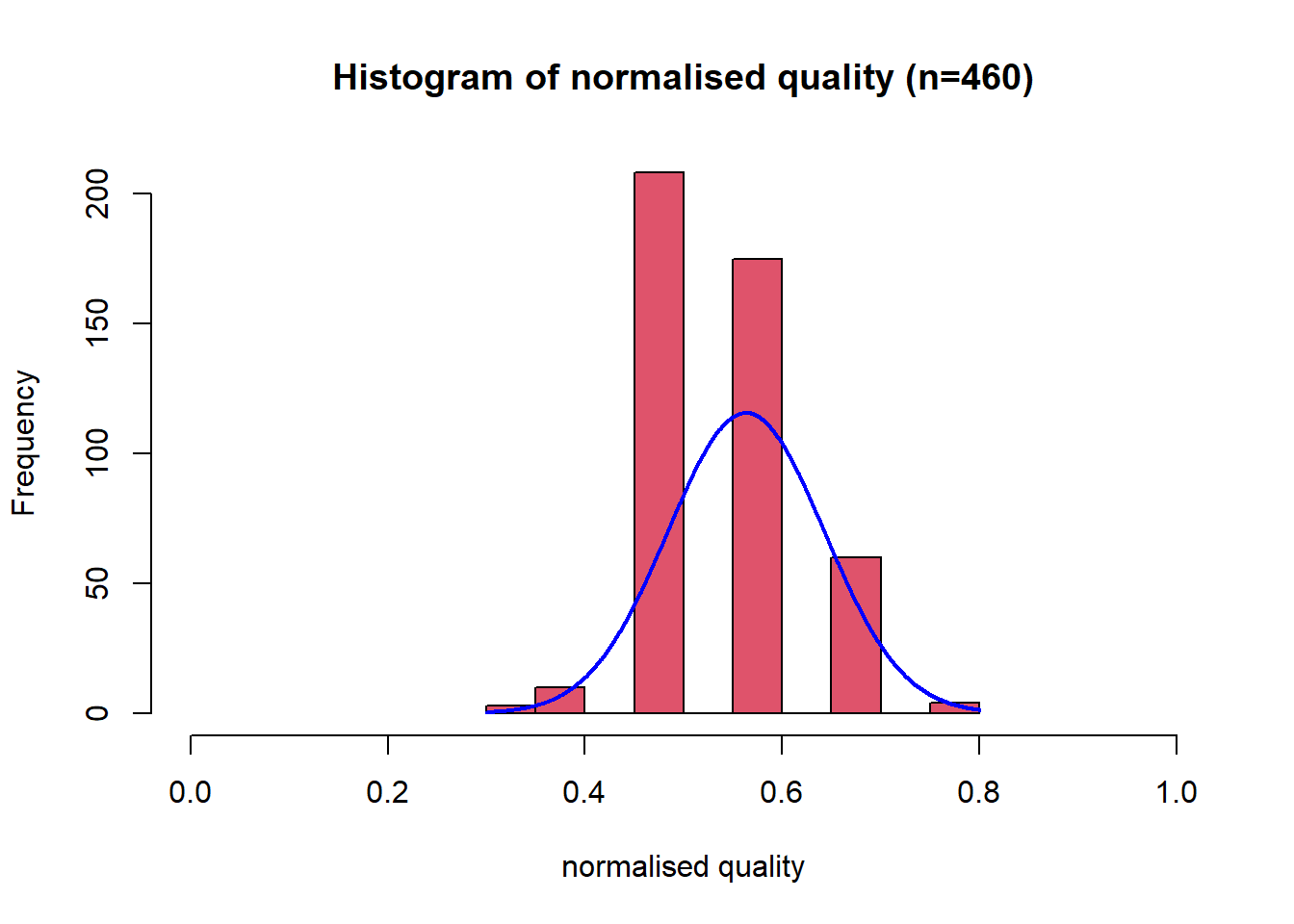
This distribution has some right skew, with a low skewness value of 0.28. Since the distribution already looks normal no data transformation was applied. The variable was then scaled between 0 and 1 using a min-max function. Using the range of possible values 0-10. This variable is the dependent variable, so no negation was required.
# Transform - no transformation applied to this variable
data.transformed[,5] = variables_for_transform[,5]
skewness(data.transformed[,5])[1] 0.2796584name5 = sprintf("%s", y.name)
title5 = sprintf("(Histogram of %s (n=%s))", y.name, subset.num_samples)
hist(data.transformed[,5],xlab=name5, breaks=8, main=title5, col=colours[I[5]])# We know the quality could range between 0 and 10, so use those values here.
min_y = 0
max_y = 10
# Normalise between 0 - 1
data.transformed[,5] = minmax(data.transformed[,5], min_y, max_y)
name5s = sprintf("normalised %s", y.name)
title5s = sprintf("Histogram of normalised %s (n=%s)", y.name, subset.num_samples)
plotNormalHistogram(data.transformed[,5], xlab=name5s, breaks=8, main=title5s, col=colours[I[5]],xlim=c(0,1))# Save this transformed data to a text file
write.table(data.transformed, "stephens-transformed.txt")Build models and investigate
A Weighted Arithmetic Mean (WAM), Weighted Power Mean (WPM) and Ordered Weighted Average (OWA) aggregation functions are fitted to the data for analysis and prediction using the AggWAfit R library of James (2016)
source("AggWaFit718.R")
# Replace previous matrix with the one loaded from disk
data.transformed = as.matrix(read.table("stephens-transformed.txt")) # import saved data
# Get weights for Weighted Arithmetic Mean with fit.QAM()
fit.QAM(data.transformed,output.1="output_QAM_AM.txt",stats.1="stats_QAM_AM.txt",g=AM,g.inv=invAM)
# Get weights for Power Mean p=0.5 with fit.QAM()
fit.QAM(data.transformed,output.1="output_QAM_PM05.txt",stats.1="stats_QAM_PM05.txt",g=PM05,g.inv=invPM05)
# Get weights for Power Mean p=2 with fit.QAM()
fit.QAM(data.transformed,output.1="output_QAM_QM.txt",stats.1="stats_QAM_QM.txt",g=QM,g.inv=invQM)
# Get weights for Ordered Weighted Average with fit.OWA()
# Note that in AddWaFit the weights don't correspond to the variables but their relative sizes.
fit.OWA(data.transformed,output.1="output_OWA.txt",stats.1="stats_OWA.txt")Model Results
| Metric | WAM | WPM (0.5) | WPM (2) | OWA |
|---|---|---|---|---|
| RMSE | 0.1403 | 0.1525 | 0.1279 | 0.1204 |
| Avg Abs Error | 0.1187 | 0.1258 | 0.1070 | 0.0985 |
| Pearson | 0.3373 | 0.2823 | 0.3857 | 0.4130 |
| Spearman | 0.3602 | 0.3179 | 0.3973 | 0.4217 |
Model Weights
| Weight | WAM | WPM (0.5) | WPM (2) | OWA (orness=0.72) |
|---|---|---|---|---|
| w1 | 0.1589 | 0.0290 | 0.2346 | 0.0590 |
| w2 | 0.3413 | 0.3772 | 0.3241 | 0.2098 |
| w3 | 0.4750 | 0.5632 | 0.3233 | 0.2537 |
| w4 | 0.0247 | 0.0306 | 0.1179 | 0.4775 |
Model Insights
-
Importance of each variable
-
From the WAM and WPM models
- The chlorides (32-37%) and total sulfur content (32-56%) are the most important variables. A majority of the models weight citric acid (3-23%) as more important than alcohol (2.5 to 11%).
OWA weights correspond with each input’s relative size, not the input’s source. The orness value of 0.72 suggests more weight is allocated to the higher inputs. The last weight indicates approximately 48% of the weight is allocated to the largest input.
-
The OWA model best fits the selected data, giving the lowest RMSE and highest correlation coefficients. On average, the prediction is out by ~ 10%
Use Model for Prediction
new_input <- c(0.8, 0.63, 37, 2.51, 7.0)
new_input_for_transform <- new_input[c(1,2,3,5)] # choose the same four X variables as in Q2
# Note: some of these values are outside the min/max that were used when transforming the data before fitting.
# It was recommended to just use a new min/max for these variables here incorporating the values from new_input.
# Transforming the four variables in the same way as in question 2
new_input_transformed = new_input_for_transform
#############
# citric acid
#############
# Transform
new_input_transformed[1] = new_input_for_transform[1]^0.75
# Normalise between 0 - 1
new_input_transformed[1] = minmax(new_input_transformed[1], min(c(min_1,new_input_transformed[1])), max(c(max_1,new_input_transformed[1])))
###########
# chlorides
###########
# Transform
new_input_transformed[2] = -new_input_for_transform[2]^-1
# Normalise between 0 - 1
new_input_transformed[2] = minmax(new_input_transformed[2], min(c(min_2,new_input_transformed[2])), max(c(max_2,new_input_transformed[2])))
# Negate
new_input_transformed[2] = negation(new_input_transformed[2])
######################
# total sulfur dioxide
######################
# Transform
new_input_transformed[3] = log(new_input_for_transform[3])
# Normalize between 0 - 1
new_input_transformed[3] = minmax(new_input_transformed[3], min(c(min_3,new_input_transformed[3])), max(c(max_3,new_input_transformed[3])))
# Negate
new_input_transformed[3] = negation(new_input_transformed[3])
######################
# alcohol
######################
# Transform
new_input_transformed[4] = -new_input_for_transform[4]^-2
# Normalize between 0 - 1
new_input_transformed[4] = minmax(new_input_transformed[4], min(c(min_4,new_input_transformed[4])), max(c(max_4,new_input_transformed[4])))
# Applying the transformed variables to the best model selected from Q3 for Y prediction
# Weights from best fitting model (OWA)
Xweights = c(0.0590,0.2098,0.2537,0.4775)
y_pred= OWA(new_input_transformed, Xweights)
# Reverse the transformation to convert back the predicted Y to the original scale and then round it to integer
######################
# quality
######################
# Normalise between 0 - 1
predicated_quality = round(y_pred*(max_y-min_y) + min_y)Prediction Example
| Variable | Raw | Transformed |
|---|---|---|
| Citric Acid | 0.8 | 1 |
| Chlorides | 0.63 | 1 |
| Total Sulfur Dioxide | 37 | 0.47 |
| Alcohol | 7.0 | 0 |
Predicted Quality: 8 (too high) - The quality prediction is too high and, therefore, unreasonable - High orness means only a few high inputs would give a high output (quality)
Best conditions for higher-quality wine
- Higher values for the inputs: citric acid, negation(chlorides), negation (total sulfur dioxide), alcohol
- Higher – citric acid and alcohol
- Lower – chlorides and total sulfur dioxide
Limitations, Ethics & Privacy
-
Limitations of the fitting model
- Correlation coefficients are weak
- OWA doesn’t tell you the importance of the variables
- The model is only as good as the training data
- The predicated value was beyond the training data set for 3 of the 4 variables
The data does not raise privacy concerns as it does not contain information about grape types, wine brands, wine prices, etc. Only de-identified data is used.
-
Professional ethics
- Transparent about the methods used to analyse the data
- R script provided that documents all assumptions and steps
- Raw and transformed data is available
References
Cortez P, Cerdeira A, Almeida F, Matos T and Reis J (2009) ‘Modeling wine preferences by data mining from physicochemical properties’. Decision Support Systems, 47(4):547-553.
Csárdi G, Berkelaar M (2024) lpSolve: Interface to ‘Lp_solve’ v. 5.5 to Solve Linear/Integer Programs [R package], v5.6.23, accessed 3 April 2025. https://github.com/gaborcsardi/lpSolve
Mangiafico S S (2025) rcompanion: Functions to Support Extension Education Program Evaluation [R package], v2.5.0, Rutgers Cooperative Extension, accessed 3 April 2025. https://CRAN.R-project.org/package=rcompanion/
Meschiari S (2024). latex2exp: Use LaTeX Expressions in Plots [R package], v0.9.8, accessed 3 April 2025. https://www.stefanom.io/latex2exp/.
Meyer D, Dimitriadou E, Hornik K, Weingessel A and Leisch F (2024) e1071: Misc Functions of the Department of Statistics, Probability Theory Group (Formerly: E1071), TU Wien [R package]. v1.7-16, accessed 3 April 2025. https://CRAN.R-project.org/package=e1071.
James, S (2016). AggWAfit R library. 10.13140/RG.2.1.1906.9688.